Types of corrosion; Galvanic corrosion
Galvanic corrosion (corrosion of dissimilar metals, incorrectly called electrolysis); It is one of the most important types of corrosion.
This type of corrosion refers to the destruction of two different substances in an electrolyte and a corrosive environment. This happens when two (or more) different metals come into electrical contact underwater.
When a galvanic (bimetallic) compound is formed, the metal that corrodes faster is the anode. And the metal that is more resistant to corrosion is called the cathode.

When contact is made with a different metal, the corrosion rate of both metals changes. In galvanic corrosion, corrosion of the anode metal stops faster and corrosion of the cathode metal stops more slowly.
Galvanic bonding is the basis of many corrosion monitoring techniques. The driving force of corrosion is the difference in the potential difference between the two metals.
History of Galvanic Fittings
This section examines the history of the galvanic connection is an example of the types of corrosionis discussed.
Bimetallic propulsion was discovered in the late eighteenth century by Luigi Galvani in a series of experiments. In these experiments, a frog’s muscles and nerves were exposed to a bimetallic conductor and contracted.
This principle was later put into practice by Alessandro Volta, who built the first electric cell (or battery) in 1800.
A number of the two types of metal disks were separated by cardboard disks impregnated with acid or salt solution. This, which became the basis for the development of modern batteries, was a remarkable scientific discovery. Because this method is known as the first method to create a stable electric current.
This principle was designed to protect metal structures in the early nineteenth century. The original open design was done by Humphrey Davy and Michael Faraday.
Corrosion of metals such as zinc, magnesium or aluminum is a widespread method for the cathodic protection of metal structures.
In the following , you will get acquainted with a number of other types of corrosion.
Content related to types of corrosion: Corrosion of metals and its aggravating factors
What happens in all types of corrosion; Galvanic corrosion
Now we want to know how galvanic corrosion is different from other types of corrosion , and also what happens in thistype of corrosion.
In a bimetallic compound, the more active materials become the corrosion anode. They also tend to corrode faster than when they are alone and not combined with anything. More noble materials act as cathodes in tuberculosis.
Galvanic corrosion can be one of the most common types of corrosion and also one of the most destructive.
By measuring the corrosion potential of a material, its relative nobility can be predicted. The EMF series is designed to estimate the potential difference between the two metals.
The small ratio of anode to cathode is an unpleasant event. Because in this case, the galvanic current is concentrated on a small anodic region.
Under these conditions, the anode loses its thickness faster. Galvanic corrosion cells can be created on a macroscopic or microscopic level. At the microstructural level, different phases or other microstructural properties can be exposed to galvanic currents.
Types of corrosion; Groove corrosion
Groove corrosion is a type of thread corrosion. Groove corrosion can be found in grooves or protected surfaces where stagnant solution is present. This is one of the most common and at the same time the most harmful types of local corrosion. Because it occurs in alloys such as stainless steel, which usually show complete resistance to corrosion.
Groove corrosion occurs in areas that are not immediately visible or inaccessible. Therefore, corrosion of the groove may lead to sudden metal failure in the device.
The grooves create a chemical environment that performs differently from free surfaces. This speeds up the corrosion process. This environment retains moisture and absorbs contaminants, as well as concentrates corrosion products and removes oxygen.
In most cases, groove corrosion occurs in near-neutral solutions. In it, dissolved oxygen reacts with the cathode.
Factors that may create grooves for corrosion
1) Structure geometry
- Riveted pages
- Welded structure
- Threaded connections
2) Metal contact with non-metallic solids
- Plastic
- Rubber
- Glass
3) Sand deposits
Permeable soil or corrosion products on the metal surface (a type ofgroove corrosion called a sediment attack).
4) Reactive components go from a higher energy state to a lower state
Groove corrosion theory requires energy. During corrosion, the reactant components move from a higher energy state to a lower state, releasing the energy required for the reaction.
In dry corrosion, metal and oxygen combine to produce oxides on the surface. This reaction leads to a compound (oxide) at a lower energy level.
The oxide layer protects the metal against oxygen. The oxide does not react with oxygen in the air or metal. This barrier makes it difficult for oxygen to contact the metal in the air. Eventually it thickens so much that the movement of electrons and ions stops throughout it.
The metal will be more resistant to corrosion if the oxide layer does not crack or is removed.
The mechanism of the Fontana model describes the types of groove corrosion. This model consists of four stages.
The Fontana mechanism, which describes the types of groove corrosion, includes the following steps:
Corrosion usually occurs inside and outside the groove:

Positively charged metal ions with –OH have an electrostatic equilibrium.
- At this stage, the cathodic reaction inside the groove consumes most of the available oxygen.
- –Cl and –OH are dispersed in the groove to maintain the minimum unreleased energy and metal chloride is formed.
Hydrolysis of metal chloride reduces pH and ![]() .
.
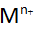 ions are more adsorbed to –Cl. This lowers the pH inside the groove. The metal dissolves faster and produces more
ions are more adsorbed to –Cl. This lowers the pH inside the groove. The metal dissolves faster and produces more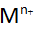 ions, which will lower the pH.
ions, which will lower the pH.
The following figure shows the mechanism of active and inactive corrosion types and their related anodic and cathodic reactions in groove corrosion.

Types of corrosion; Stress Corrosion Cracking (SCC)
Another type of corrosion, stress cracking (SCC), is a fracture of the alloy exposed to the environment. Which occurs due to a relatively low but constant tension. A synergistic action of corrosive environment and tensile stress on the material is required to create SCC.
The figure below shows the three factors required to create SCC, which is a type of corrosion.

When stainless steels are in contact with the wet environment, the passive layer contains chromium oxide which is hydrated (Sedriks 1986). The uniform corrosion rate of stainless steel in this case will be very low due to the formation of the patio film.
However, the patio layer may be present locally at points where the Passive layer is weakened. Such as sediments, sediments and granules or spots of solution that occur due to the accumulation of invasive separation ions. For example, chloride ions accumulate in the surface roughness, causing the patio layer to break.
Passive layer failure
This local failure of the Passive layer leads to a variety of localized corrosion. For example, cavity corrosion and stress cracking.
Due to the difference in the chemical composition of stainless steels, the composition of the Passive layer also changes for different types of stainless steels. However, chromium still forms a large part of the Passive layer, which protects the surface layer.
Working conditions over a period of time may give rise to stress cracking (SCC). Therefore, a view has now been taken that states that no specific environmental composition is necessary to cause stress cracking (SCC).
The tensile stress required for stress cracking (SCC) can be less than the stress of the macroscopic product. However, residual stresses due to welding / heat pressures and high pressures can lead to stress cracking (SCC).
This is possible if the pressure due to the strength of the alloy is locally exceeded in the material. Types of SCC cracking can be intergranular stress corrosion cracking (IGSCC), or transgranular stress corrosion cracking (TGSCC). Or display a combination of both.
Significant progress has been made in the field of stainless steel stress cracking in the last two decades. In particular, understanding grain boundary engineering and the function of the structure that affects stress cracking.
Types of corrosion; CO2 corrosion
CO2 corrosion can be mentioned as one of the most important types of corrosion. Carbon dioxide (CO2) is found in oil and gas fields at different concentrations.
Of the types of CO2 corrosion, dry CO2, in the supercritical gas or fluid stage, does not corrode metals and alloys. However, in the presence of liquids produced containing water, there is a possibility of severe corrosion of the infrastructure due to the formation of carbonic acid.
Factors affecting CO2 corrosion
- CO2 concentration (and other components such as H2S)
- Water chemistry
- Operational conditions
- Type of material
Types of corrosion CO2; Corrosion in the oil and gas industry
The economic impact of CO2 corrosion on the oil and gas industry has long been known. Many methods have been used to reduce the effect. However, due to the increase of new methods with increasing temperature, pressures and different fluid compositions, the corrosion performance of the material must be re-evaluated.
CO2 corrosion is a common problem in oil and gas production.
Analysis and extraction of models
Despite systematic efforts to analyze and extract appropriate models for predicting this type of corrosion, not all aspects are clear and there is ambiguity about the mechanism and parameters that affect it.
In addition, existing and accurate information models are not detailed about highly corrosive environments.
Dry carbon dioxide; Types of CO2 corrosion
Dry carbon dioxide is not corrosive at normal temperatures of oil and gas systems. But after dissolving in the aqueous phase, it causes an electrochemical reaction between the steel and the environment.
Hydrocarbon liquids; Types of CO2 corrosion
CO2 gas has a very high solubility in water and salt water and its solubility is slightly higher in hydrocarbons.
Hydrocarbon liquids are generally produced in contact with the aqueous phase. Also, in many cases, hydrocarbon reservoirs contain significant amounts of CO2. As a result, CO2 dissolves in the aqueous phase of the hydrocarbon product, and this aqueous phase will corrode the carbon steel.
Mechanisms of CO2 corrosion
Types of corrosion; Mechanisms of CO2 corrosion.
The corrosion mechanisms of carbon dioxide are complex. Initially, CO2 gas is dissolved in water to produce bicarbonate ions, carbonic acid, and hydrogen ions. These components have the ability to move towards metal surfaces and help with the reduction reaction.
Reactions, including bicarbonates, lead to higher corrosion rates than expected from acidity. At temperatures below 60 ° C and pH values below 4, corrosion is controlled by the production of iron ions with a surface covered with iron carbonate.
In many cases, a semi-protective shell of iron carbonate is formed at temperatures above 70 ° C and the reaction is cathodically controlled. This reduces the corrosion rate.
It is also controlled by partial CO2 pressure, temperature and pH (control of bicarbonate ion concentration), and fluid conditions. The maximum corrosion rate is reported to be around 70 ° C. (Kermani and Morshed, 2003; Schmidt, 2015)
Some other different mechanisms have been proposed for this type of corrosion. They all contain carbonic acid or bicarbonate ions, which are formed by dissolving CO2 in water. This results in higher corrosion than expected in strong acids at the same pH.
Dissolving CO2 in water produces carbonic acid, which is weaker than mineral acids. (Nyburg, 2002; Pots, 1995)
The carbonic acid reaction steps are as follows. (Kermani and Morshed, 2003; Schmidt, 2015; Pots, 1995):
The mechanism introduced by De Waard is also well known. (de Waard et al.، 1991؛ de Waard، 1993؛ George et al.، 2004).

Regarding the mechanism of CO2 corrosion, there is a debate about the timing of the presence of other soluble components in the corrosion reaction. The amount of corrosion depends on the partial pressure of CO2. Because this parameter determines the pH and concentration of soluble components.
In fact, the whole chain of electrochemical reactions is more complex than mentioned. Depending on which stage controls the amount of corrosion, the dependence of the corrosion reactions on the pH and CO2 of the solution will vary. (Nyburg, 2002; Pots, 1995).
Types of corrosion; Local corrosion caused by CO2
In various types of corrosion, localized corrosion due to CO2 (sweet corrosion) is a serious concern in relation to oil and gas pipelines. Depending on the stagnation / flow state and fluid velocity, local CO2 corrosion is divided into three groups including cavity, mesa attack, and local flow corrosion.
Cavitation occurs at low fluid velocities in the point temperature range. As the temperature and partial pressure of CO2 increase, the sensitivity to cavitation increases. (Schmidt, 2015; Schmidt and Feynen, 2000; Schmidt and Engels, 1998).
Types of corrosion; Mesa attack corrosion
Mesa attack corrosion is a type of sweet corrosion that occurs at low to medium fluid velocities. Where the shell is composed of iron carbonate is unstable under these conditions and can not withstand the fluid. (Kermani, 1997).
Localized corrosion due to flow begins when the fluid moves rapidly above the critical point, starting with the cavity or Mesa attack and accelerating with turbulence caused by cavities and bridges.
If the carbonate shell destroys and removes the protective membrane, it will cause local movements and stresses along it. After removal, the high velocity of the fluid shell does not allow the membrane to regenerate.
What is hydrogen brittleness?
Usually hydrogen can only combine with metals in the form of hydrogen atoms or ions. Thus, hydrogen, which is dispersed as a gas, has a molecular form and is not absorbed by metals at ambient temperature.
However, as the temperature increases, the molecules tend to decompose into separate atoms. This process allows atoms to combine with materials at certain temperatures. For example, during oil refining or heat treatment.
In molten materials, higher adsorption of atoms is observed, which means that processes such as casting and welding can provide special opportunities to combine hydrogen with metallic materials.
Hydrogen ions are also produced by reactions associated with processes such as corrosion, plating, and cathodic protection. As a result, there is ample opportunity to combine hydrogen with metal components.
Cracks associated with hydrogen brittleness come in many forms depending on the conditions under which they occur, including the following.
Cold cracks and delayed cracks
Another type of corrosion includes cold cracking and delayed cracking (hydrogen cracking). This part is created when the metal and workpieces cool down after welding the steels.
Hydrogen-induced Cracking (HIC) or Hydrogen Pressure-induced Cracking (HPIC):
Apart from its general meaning, it refers to the specific morphology of cracks in steel pipelines and reservoirs that absorb hydrogen.
Hydrogen-induced Stress Cracking (HISC):
Cracking This type of cracking refers to cracking during service in duplex steels, but has recently become more common.
Types of corrosion; Environmentally-Assisted Cracking (EAC)
This cracking can occur due to the interaction between the components and the environment, and hydrogen is one of the factors affecting this type of cracking.
Disbonding
These terms refer to the fragmentation of the weld pool of tanks used to process high-temperature hydrogen gases.
Stress Corrosion Cracking (SCC)
Some of the specific mechanisms of this phenomenon are related to the interaction with hydrogen.
Sulphide Stress Cracking (SSC)
Corrosion in environments containing hydrogen sulfide can cause hydrogen adsorption and cracking. The specific crystal structure of metals is also important because it affects the rate of hydrogen diffusion and deformation.
Accordingly, ferritic steels are more sensitive to hydrogen brittleness than alloys with different crystalline structures, such as austenitic stainless steels, nickel alloys, and aluminum alloys. However, it is clear that hydrogen can make most engineered alloys brittle.
When this happens, the fragility of hydrogen can reduce ductility and load-bearing capacity, which will eventually lead to cracking and brittle damage.
Types of corrosion; Cavitation corrosion
Cavitation corrosion is another type of corrosion that occurs when a bubble forms and bursts in a liquid. Cavitation corrosion is also known as bubble erosion.
However, there is not much difference between cavitation corrosion and bubble erosion. During cavitation corrosion, both corrosion and cavitation degradation occur, but during bubble erosion, only cavitation degradation occurs.

Cavitation corrosion occurs in equipment such as hydraulic turbines, turbine blades, propellers of aircraft that can land and move in water, and pump blades.

To learn more about this type of corrosionand to understand the formation and collapse of cavities in the system, consider water containing cylinders equipped with a solid piston. When the piston is pulled, the volume inside the cylinder increases and the pressure decreases. As the pressure decreases, the water begins to boil, causing bubbles to form at room temperature.
As the piston moves inward, the volume decreases, the pressure increases, and eventually the bubbles collapse.
This process is repeated many times and at high speed in turbines, propellers and blades, during which bubbles form rapidly and disappear immediately. Bursting bubbles destroy the protective layers on the metal surface.
The unprotected surface of the metal corrodes and regenerates. The process of bubble regeneration and disintegration destroys the layers that have just formed on the surface.

In the absence of surface protective layers, the formation and disappearance of bubbles is accompanied by deformation of the plastic, which causes metal particles to move away from the surface.
Corrosion expands cavitation. Thus, cavitation corrosion has become a synergistic process that includes mechanical processes (bubble formation and collapse) and electrochemical processes.
Cavity damage can be controlled by minimizing the possibility of bubble formation and collapse. This is made possible by the design of hydrodynamic systems with lower pressure differences.
In the case of a pump that is cavitated, the standard method is to increase the system pressure effectively to prevent bubbles from forming at low pressure points in the system. In addition, hydrodynamic systems must be made of materials that are resistant to corrosion and mechanical damage.
Due to the choice of materials, it should be noted that malleable materials are more resistant than brittle materials. Substances that wear out later are also more resistant to cavitation corrosion.
As the particle size of the material decreases, the corrosion resistance of the cavitation increases. Rough surfaces provide a better substrate for bubbles than smooth surfaces.
The surfaces of the materials are protected by a coating, which is usually made of rubber or plastic. When using the coating, it should be noted that if there is a gap between the coating and the metal, it can be a good place for cavities to form.
Cathodic protection and the use of inhibitors and corrosion control reduce the rate of cavitation corrosion. But it has no effect on damage caused by mechanical forces.
Cavity corrosion
Cavity corrosion is a very complex phenomenon. Despite numerous studies on thetypes of corrosion over the past century, it is still not completely transparent. However, the conditions that cause the initiation, expansion and slowing down of the pitting corrosion process are somewhat known.
Cavity corrosion mechanism
Several consistent hypotheses have been proposed to explain the mechanism of pitting corrosion, the main steps of which are shown in the following figure:

The mechanism of pitting corrosion can include the following steps:
1- Passive layer failure
2- Cavity development
3- Re-passivation of the cavity
These parameters depend mainly on the state of the oxidized layer, the structure of the metal in which the intermetallic particles play a major role, the nature of the surrounding aqueous medium, and its chloride content.
tip: Groove corrosion can be interpreted as pitting corrosion. But the difference is that groove corrosion occurs in the middle surfaces protected from liquids and there is little possibility of liquid penetration. Cavity corrosion, on the other hand, occurs on surfaces that come in direct contact with the wet environment.
The beginning of the hole
According to Richardson, the oxidized layer is constantly being damaged and repaired. In non-invasive environments, it can be repaired immediately. However, in the presence of a solution containing aggressive ions, especially chlorides, the access of these anions to the surface prevents its repair. The reactivity of the oxidized layer is then lost.
For many researchers, the onset of cavities is due to the penetration of chloride into the oxide layer; Either because those layers have innumerable defects, which become the entry points of chlorides, or because they are positively charged in aqueous solution.
These defects are usually accompanied by physical inhomogeneities such as the presence of intermetallic components and particles on which the oxide layer is thinner, Physical surface changes (eg, minor scratches) are caused by mechanical movements, polishing, stone cutting, or passing through shaping tools.
The onset of cavities is a very rapid phenomenon and difficult to observe because it occurs on a micrometer scale and, moreover, is random in nature and its exact location cannot be predicted.
There is no latency to start cavities. Warner and Schmidt were able to observe under an atomic force microscope (AFM) on the AA2024 alloy that cavities formed in less than 2 minutes in sodium chloride solution. These micropores, 0.1 to 1 μm in diameter, were formed in large numbers with an ![]() density.
density.
Passive layer failure
Several models have been proposed to explain the failure of the passive layer, including the following:
- Infiltration of anions into the oxide layer
- Rupture of the oxide layer
- Adsorption of anions by the oxide layer
The breakdown of the passive layer allows the ions to reach the oxide / metal layer interface where they dissolve the metal.
Two explanations for influence are provided:
Existence of a very strong attractive electric field in the oxide layer, which contains 106 to 107 V.cm−1 and a positive charge on the surface of the oxide layer, respectively.
According to McCafferty, the interaction with water molecules forms a layer of hydroxyl+ AlOH2 groups on the surface of the oxide film with a thickness of 0.5 to 0.8 nm.
The density of these hydroxyl groups is between 6 and 20 nm.
The isoelectric point of the oxide layer occurs on aluminum at pH 9.5. Thus, for neutral solutions with lower pH that are more compatible with natural environments, the oxide layer has a positive charge and naturally −Cl chloride ions and other anions such as sulfates −SO42 Attracts.
Oxide layer failure occurs for reasons such as the presence of vacancies in the oxide. The voids expand by moving to the metal / oxide surface to capture Al3 + cations from the metal. If the share of these cations is insufficient, they can form cavities, which will become places to create cavities.
In order for aluminum to dissolve on the surface of the oxide / metal layer, water molecules must also be able to penetrate through the cracks or pores of the oxide layer. These areas are around surface heterogeneity and even mechanical shear due to movement, shaping and polishing.
Adsorption is a model that has been considered by many authors over time.
According to Szklarska Smialowska, chloride ions are adsorbed on the oxide layer or combined in both. Chlorides easily penetrate the oxide layer due to their small size.
Their radius is 18.1 nm, which is slightly larger than the oxide layer (14 nm). They can move through empty oxygen spaces.
Several research methods, including autoradiography, have shown the adsorption of chlorides on the oxide layer. The critical chloride concentration threshold is unknown. However, if such a threshold exists, the chloride concentration should be at least ![]() .
.
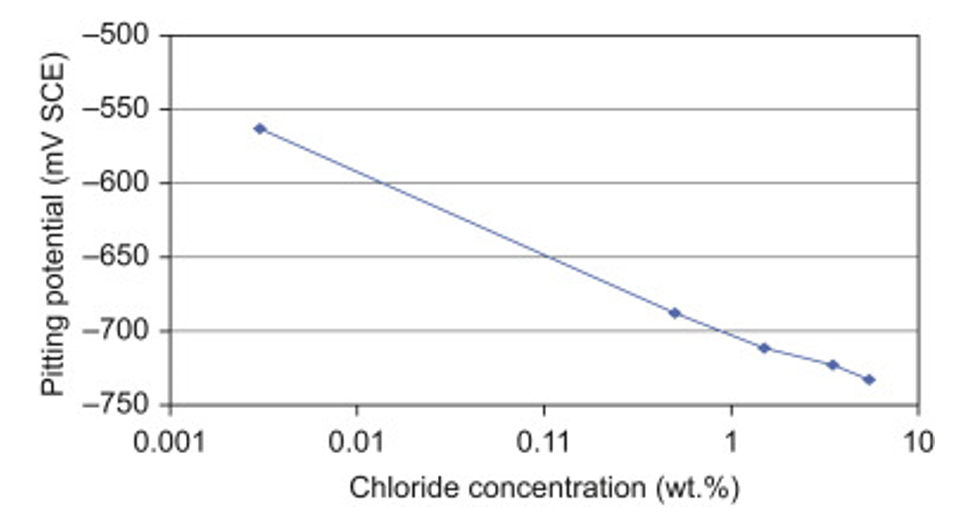
According to Zaid, there is a relationship between chloride concentration and cavity potential. The effect of chlorides decreases with increasing concentration. This relationship is especially clear above 1% by weight (1% wt).
On the official website of Naftan Payesh Company, in the section of specialized corrosion magazine, you will find materials related to “types of corrosion“.
Stay with us.
Source
https://www.nace.org/resources/general-resources/corrosion-basics/group-1/galvanic-corrosion
https://soar.wichita.edu/bitstream/handle/10057/917/grasp%20216.pdf
https://www.sciencedirect.com/topics/materials-science/pitting-corrosion
https://www.sciencedirect.com/topics/engineering/cavitation-corrosion
https://www.twi-global.com/technical-knowledge/faqs/what-is-hydrogen-embrittlement
https://www.sciencedirect.com/science/article/abs/pii/S1875510018303366













