Microbial corrosion
Microorganisms are ubiquitous in nature and grow rapidly in air, soil and water. Organisms involved in the three processes of biopollution, biodegradation and biodegradation are often abundant because they are particularly tolerant of environments with drastic changes such as changes in pH, temperature, pressure and metal concentrations.
The basic definition of corrosion is the degradation or degradation of metals and alloys in the environment using chemical or electrochemical methods.
Involvement of microorganisms in metal corrosion can be based on biological corrosion, Microbial corrosion, or more specifically microbial (or microbially induced) corrosion (MIC), can be defined as an electrochemical development involving a microorganism that can accelerate anodic and / or cathodic reactions, or Facilitate.
Uniform and microbial corrosion are based on electrochemical reactions. The role of microbes in corrosion is often considered as electrochemical catalysts. Microbial corrosion can begin and persist in aerobic, anaerobic, acidic, neutral, or alkaline conditions. To fully understand the mechanisms of MIC, complementary maps of biological, chemical, and electrochemical agents must be considered.
MIC is not limited to one corrosion mechanism and generally cannot be classified into different types of corrosion. MIC can lead to localized attacks such as cavitation, groove corrosion, alloying (galvanic attack), by corrosion deposition, and can also accelerate galvanic corrosion, stress corrosion, and hydrogen cracking.
MIC occurs in all environments, especially in terrestrial, freshwater and seawater environments and is estimated to be responsible for more than 30% of all corrosion damage.
Biodegradation can be defined as the cause of changes in the undesirable properties of materials that lead to their decay due to the activity of microorganisms. Basically, biodegradation refers to non-metallic materials such as wood, rubber, fuels, lubricants, paints, building materials such as cement, concrete and mica, and polymer coatings. In these cases, the failure is not electrochemical, but related to the role of microorganisms.
The terms biodegradation and biodegradation are fundamentally different. Because the first case shows the corrosion of metal (any alloy) caused by electrochemical devices in the presence of microbes, while the second case includes microbial degradation and the destruction of non-metallic materials. Biodegradation is related to biodegradation.
Bio-pollution refers to the adverse adhesion and accumulation of microorganisms and microorganisms on submerged structures (materials), such as seawater. Unlike non-living sediments such as sediment, organic sediment or particles, biological sediment contains micro- and macro-organisms as liquid material that are firmly attached to surfaces.
Microbial accumulation in the substrates along with the products of metabolism appear as biofilms whose role in the MIC must be clearly defined. Initially, fine dust (due to the adhesion of microorganisms such as bacteria) is done, followed by coarse deposition (adhesion of fungi, algae, sea urchins, oysters, etc.). In the bacterial groups present in biofilms, sulfate-reducing bacteria (SRB) play the largest role in corrosion of steel (such as pipelines).
SRB is widely involved in corrosion in the oil and gas industry. Other organisms involved include metal oxidizing bacteria (MOB), iron oxidizing bacteria (IOB), methanogens and acid-producing bacteria (APB), and fungi. The industrial importance and greatness of bio-sediment and MIC can be seen from the following tables.
| Equipment involved | Industry |
| Cooling pipes (condensers), underwater pipelines, heat exchangers (stainless steel and carbon steel, copper and aluminum alloys) | Hydro and nuclear power plants |
| Underground equipment and materials for metal engineering and
Ferrous and non-ferrous alloys |
Mining and metallurgy operations |
| Condensers, heat exchangers, pipes and storage tanks | chemical industry |
| Many equipments (stainless steels) | Paper and pulp industries |
| Heat exchangers, oil breakers, emulsions and lubricants | Metalworking |
| Concrete structures in marine, freshwater and groundwater conditions, stairs, buildings | Construction and sewage |
| Aluminum alloys and steels, oil and gas pipelines, water injection in oil tanks | Oil and gas industry |
| Aluminum fuel tanks | Aerospace section |
| Stagnant state, copper and steel pipes, hot water pipes | Wastewater and drinking water treatment facilities |
| Water pipes and hoses, use of untreated water | Fire protection systems |
Table 1- MIC relationship in industrial environment
| Alloy involved | Type of equipment |
| Aluminum brass, nickel copper, alloys, stainless steels, titanium (pitting) | Heat exchanger tube |
| Stainless steels, carbon steels (pitting, knotting) | Water storage tank |
| Stainless steel welds, carbon steels (pitting, blistering) | Water pipes |
| Galvanized steels (blocking, pitting) | cooling towers |
| Stainless steels (gaps, cavities) | Pumps |
Table 2 – MIC in power plants
| Agent name | Type of agent |
| Sulfur oxidizing bacteria (Acidithiobacillus spp)
APB (inorganic sulfuric acid by Acidithiobacillus, organic acids by Bacillus spp) Desulfovibrio SRB, Desulfotomaculum, Desulfobacter IOB or MOB and metal precipitating bacteria (Galionella, Carnotrix, Lepotrix, Sphaerotilus) MRB Pseudomonas, Schwanella. Sludge-producing bacteria (Bacillus, Flavobacterium, Aerobacter, Pseudomonas) |
bacterias |
| • Cladosporium resinae
• Paecilomyces varioti • Aspergillus niger. All the fungi produce organic acids • Penicillium cyclospium |
Mushrooms |
| • Blue-green algae | Algae |
| • Interact with different groups of microorganisms | Microbial consortia |
Table 3. General classification of microbes participating in the MIC
Related microorganisms and environmental centers
The microorganisms that cause corrosion can be generally classified in Table 3.
Other effects include the association of microbial handles on the metal corrosion of the exopolymer, scale, and debris formed on metal surfaces. Such sediments create corrosion pits beneath it and slit layers of sludge.
Extracellular polymers act as a key to corrosion as part of the biofilm. The production of acid (both organic and inorganic) and the production of corrosive substances such as H2S cause holes and dissolve the metal.
Table 4 lists the environmental and biological characteristics of important MIC microorganisms.
| Alloy affected | Metabolic function | Oxygen requirement | pH | Temperature°C | Organisms |
| Iron and steel, stainless steel, aluminum, copper and zinc | Use H2 to reduce to -2S and H2S | anaerobic | 8-4 | 40-10 | Desulfovibrio |
| Iron and steel, stainless steel | Reduction to -2S and H2S | anaerobic | 8-6 | 40-10 | Desulfotomaculum |
| Iron and steel | Reduction to -2S and H2S | anaerobic | 8-6 | 40-10 | Desulfomonas |
| Iron and steel | Fe2+→Fe3+S | Aerobic | 6-1 | 35-25 | A. ferrooxidans |
| Iron, steel and reinforced concrete | S and S –2 to | Aerobic | 5-0.5 | 35-25 | A. thiooxidans |
| Iron and steel, stainless steel | Oxidation of iron and manganese and formation of tubercle | Aerobic | 10-7 | 40-25 | Gallionella |
| Iron and steel | Oxidation of iron and manganese | Aerobic | 9-7 | 35-20 | Leptothrix |
| Iron and steel, stainless steel | Oxidation of iron and manganese | Aerobic | 10-7 | 40-20 | Sphaerotilus |
| Iron and steel, Al | Some strings convert +3Fe to +2Fe. | Aerobic | 9-4 | 40-25 | Pseudomonas |
Table 4 – Biological characteristics and function of some MIC-producing microorganisms
Microorganisms cause corrosion by changing the electrochemical conditions at the surface of the metal solution, which can be modified by biofilm formation.
MIC is closely related to microbial adhesion, extracellular polymeric materials (EPS) and other products with the interaction present in the biofilm.
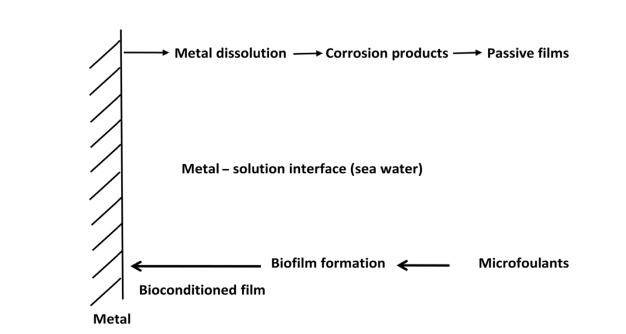
Biofouling begins immediately after the metal is immersed in aqueous solutions, and several nanometer-thin layers consisting of ions and organic and inorganic compounds are formed in the first stage. Surface ventilation facilitates bonding and microorganisms due to electrostatic charges and wetting capability.
Over time, microbial growth and EPS production facilitate biofilm development. Along with biological changes leading to biological deposition, the process of metal corrosion and corrosion product formation also occurs after the metal is immersed in an aggressive environment such as seawater.
Electrochemical corrosion and bio-deposition may occur on a similar time scale, follow opposite directions in each of these processes, as shown in Figure 1.
The bacterial cycle of sulfur in natural environments is related to MIC. Sulfur and sulfide oxidizing bacteria and SRB are involved in a number of biogenic redox reactions that lead to products such as H2S, sulfides and sulfoxy compounds.
SRB, like Desulfovibrio, reduces sulfate to sulfide and hydrogen sulfide under reducing conditions.
SRB plays an important role in the sulfur cycle. Anaerobic bacteria use sulfate as the final electron acceptor in the decomposition of organic matter, leading to the production of H2S.
The sulfide can be subsequently oxidized by the aerobic-sulfur-oxidizing chemolithotrophic acid Acidithiobacillus sp. To the element sulfur and sulfate shown in Figure 2.
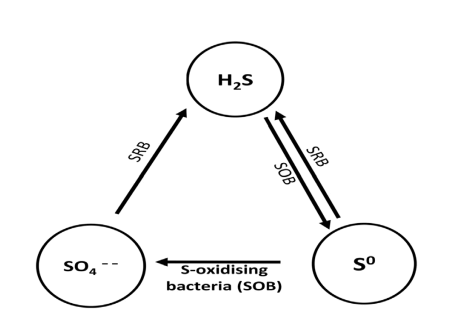
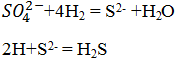 Sulfur and ferrous IOBs such as A. thiooxidans and A. ferrooxidans are aerobic acidophiles that oxidize sulfur, sulfides, and iron ions.
Sulfur and ferrous IOBs such as A. thiooxidans and A. ferrooxidans are aerobic acidophiles that oxidize sulfur, sulfides, and iron ions.
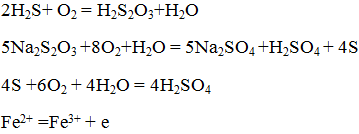
The bacterial cycles of iron and manganese are also related to MIC. Because iron and manganese oxidizing organisms are involved in microbial corrosion. Sludge-forming bacteria such as Bacillus subtilis, Bacillus cereus, Flavobacterium, Aerobacter and Pseudomonas are present in peripheral soils. Pesomonas grow in environments containing hydrocarbon sources such as petroleum oils and emulsions.
Single-celled to multi-celled species with various shapes are collected under algae. Most algae contain pigments such as chlorophyll and grow easily on wet surfaces of cooling towers, plates, and distribution systems. Many common algae, such as blue-green algae, green algae, and diatoms, produce corrosive organic acids and oxygen, causing metal corrosion.
Fungi are similar to chlorophyll-free algae. The molds are filamentous fungi while the yeasts are single-celled. Fungi involved in MIC include Aspergillus niger, Aspergillus fumigatus, Penicilium cyclospium and Cladosporium resinae.
Fungal metabolism produces organic acids such as oxalic acid, citric acid, and gluconic acids, which can cause microbial corrosion of many ferrous metals and alloys.
E and pH are environmental parameters that control electrochemical reactions in corrosion. Optimal growth and activity regions for various MIC-producing bacteria such as A. ferrooxidans, iron sulfur oxidizers, A. thiooxidans, ironoxidizing neutrophil heterotrophs, and anaerobes such as SRB are also shown in Figures E and pH.
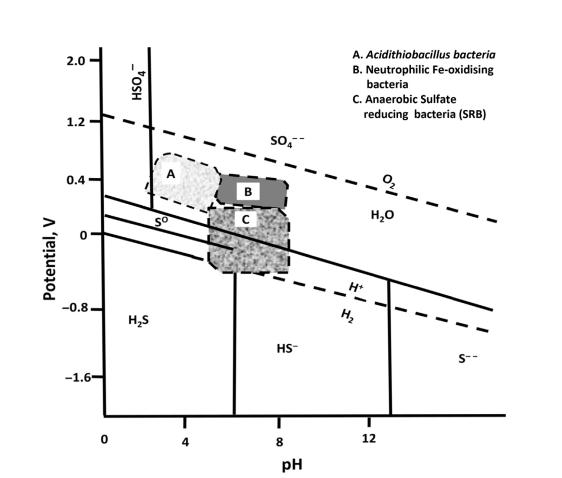
Typical E-pH corrosion diagrams made for various 2H2O_O metal systems can be modified to reflect the corrosion behavior of metals and alloys when microorganisms are also present in the environment. A typical modified pH diagram to predict iron corrosion behavior in the presence of neutrophil IOB and SRB is shown in Figure 4.
In the presence of SRB, sulfide compounds such as FeS and 2FeS are formed on iron surfaces. IOB, on the other hand, converts iron into iron oxides and hydroxides. In case of bacterial growth and metal interactions, the active, inactive and safe areas of metals can be changed from the areas initially predicted under Eh and pH.
Iron passivity can be eliminated by reducing the SRB, which causes a localized surface cavity. Microbial cavitation and groove corrosion shown by passive alloys such as stainless steels in the presence of SRB are a common example.

General mechanisms in the MIC
Anodic and cathodic electrochemical reactions, such as general corrosion, also form the basis of the MIC. Direct and indirect mechanisms may be involved in MIC processes.
Microbes, on the other hand, are directly involved in the reaction of the electrode through their metabolism in the mechanism. Indirect mechanisms of MIC include the production of corrosive microbial reactants such as organic and inorganic acids as well as ammonia, phosphides, and sulfides. There is no direct electrochemical involvement of microorganisms in indirect mechanisms.
The corrosion functions of microorganisms are very complex:
- Production of inorganic and / or organic acids, ammonia, sulfides and phosphorus compounds that create corrosive environments.
- Destruction of passive and protective layers of passive metals and alloys such as aluminum, stainless steels, chromium and nickel.
- Destruction of coatings (paint, resin), destruction of inhibitors.
- Secretion of oxidation enzymes, for example, (hydrogenase by SRB) or electrochemically active components such as cytochromes and flines.
- Production of exopolymeric compounds such as proteins and polysaccharides that can dissolve metal ions and bind to them.
- Through biofilm formation, it creates a concentration gradient such as oxygen concentration cells or metal ion concentration cells, which form anodic and cathodic regions, and with it the onset of microbial adhesion.
- Direct interference with anodic and cathodic reactions involving electron transfer – change in open circuit potentials by metabolic activity.
- Increased potential in inert metals and alloys (stainless steels) due to biofilms affecting anodic or cathodic processes.
Microbial corrosion in important alloys
There are no known metals or alloys that can fully resist biofouling and biofilm formation. The capability and sensitivity to MIC for several important ferrous and non-ferrous metals and alloys are as follows:
Copper and its alloys are commonly used in heat exchangers, pumps, valves and condensers. 90/10 and 70/30 Nickel Copper, brass, aluminum bronze and marine brass are susceptible to microbial corrosion in seawater.
Extracellular biopolymers secreted by microorganisms corrode copper alloys through blow differences, selective dissolution, and stress corrosion. Cavitation, compaction and cracking of rice and bronze under microbial activity are known in marine and industrial environments. SRB builds sulfide-rich scales on copper alloys, leading to blisters.
Although copper ions are toxic to microorganisms, copper and copper alloys are not free of biological contamination and biological corrosion. The group of bacteria Acidobiobicillus can develop higher tolerance than copper ions and dissolve the metal more effectively.
The bacteria that formed the sludge along with the corroded iron were isolated from the degraded components of nickel-copper alloy and monel pipes used in nuclear power plants. SRB corrodes pipes and copper pipes.
Bacterial ammonia produced causes stress corrosion of several copper alloys. Rice MIC in heat exchanger tubes is known by ammonia produced by nitrate bacteria.
Carbon steels are commonly used to transport water, oil and gas in basements and marine environments. Pipe formation occurs under pipes and steel pipes, which leads to reduced flow and connection problems.
Aerobic and anaerobic microorganisms attach tightly to steel surfaces, resulting in complex biofilms. Aerobic aerobic bacteria such as Galionella, Leptotrix and A. ferrooxidans are involved in causing furrow corrosion and cleft palate.
These organisms oxidize iron ions to ferric, resulting in the deposition of iron hydroxide oxide in the biofilm. Anaerobic bacteria such as SRB can also settle in the bumps formed in mild steel pipelines.
Aerobic bacteria can be involved in corrosion in several ways, such as sludge formation, oxidation of iron and sulfides, and the production of acidic metabolites. The deposition of hydrated bacterial sludge covers the metal surfaces, creating areas of diffusion and creating an environment for the subsequent growth of anaerobic organisms.
IOB oxidizes iron ions less than soluble iron ions and leads to the formation of insoluble protrusions, consisting of hydrated iron oxyhydroxides and biological metabolic products.
Steel water and oil pipes are prone to such attacks. The formation of large, durable protrusions inside steel pipes not only blocks fluid flow, but also causes severe local corrosion in the form of large cavities and grooves.
Stainless steels find wider industrial applications in nuclear power plants in freshwater and seawater environments. IOB, MOB and manganese precipitating bacteria cause corrosion of stainless steels.
The MIC of austenitic stainless steels is usually determined by drilling in the vicinity of the weld. SRB can also corrode stainless steels, ultra-stainless steels such as duplex steels and molybdenum steels.
Aluminum and its alloys The protective oxide films on aluminum and its alloys can be disrupted and destroyed by microorganisms.
2024, 7075 aluminum and alloys used in aircraft and fuel storage tanks are prone to microbial corrosion. The production of water-soluble organic / mineral acids by bacteria and fungi can lead to cracks and intergranular corrosion in aluminum.
Susceptible alloys
Aluminum-magnesium alloys (5000 series) used in marine applications are subject to perforation, intergranular corrosion, peeling and stress corrosion due to bio-sediment.
Aircraft fuel tanks and seawater components made of aluminum and its alloys are attacked by organisms such as Pseudomonas, Leptotrix, SRB, and fungi. C. resinae grows on petroleum products, kerosene, or paraffins as the only source of carbon. And brownish colonies form.
Fuel tanks, especially on ground aircraft, are severely contaminated by fungal and bacterial growth. For example, the following microorganisms have been isolated from aircraft sludge sludge, namely Pseudomonas aeroginosa, Aerobacter, Aerogenes, Clostridium, Bacillus, Desulfovibrio, Fusarium, Aspergillus, Cladosporium and Penicillium. The cavitation potential of aluminum alloys can be reduced by microbial adhesion and interaction with organic acids produced by fungi.












