What is iron corrosion?
Practically the only factor that limits the longevity of Iron is its Oxidation. In the United States, an estimated 100 billion$ is spent annually to replace Iron equipment damaged by Iron corrosion.
Iron corrosion
Iron corrosion usually occurs with the formation of Iron rust due to an electrochemical reaction in attendance of Oxygen and a salty electrolytic solution. When iron reacts with water and oxygen, iron hydroxide (II) (Fe (OH) 2) is formed.
Iron Hydroxide reacts with Oxygen to form Hydrated Iron Oxide (III) (Fe2O3 · xH2O), that known as rust.
This Oxide cannot form a protective layer on the surface of the Iron. Resembling the layer that forms on the Aluminum metal (Al2O3) and the constructed layer, separates from the metal surface.
On the other hand, the Iron Hydroxide layer peels off the surface, exposing the fresh metal to Oxygen and Water.
Both Oxygen and Water are needed to form Iron rust Iron corrosion . Iron nails placed in deoxygenated water will not corrode. Even if the immersion lasts for several weeks.
On the other hand, a Iron nail dipped in oil will not corrode . Because Oxygen cannot be present.
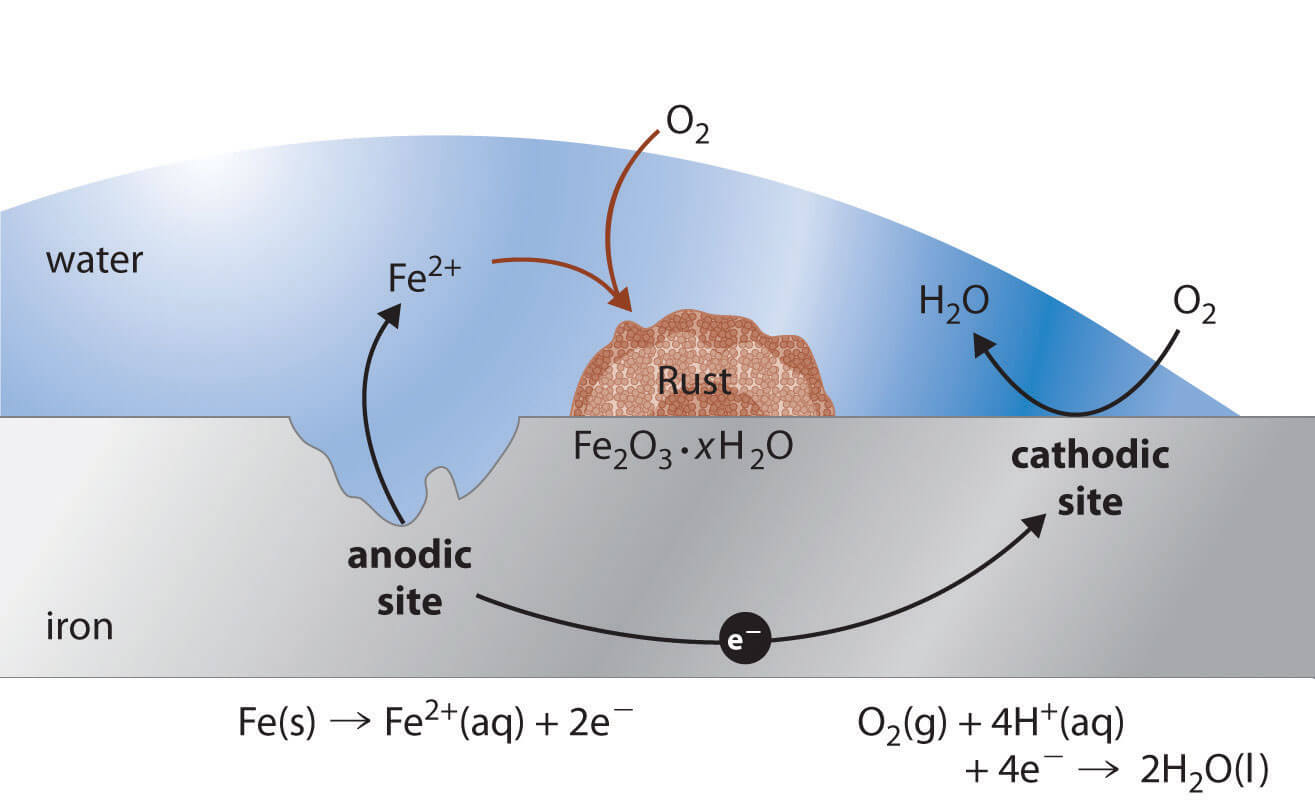
Iron is converted to Fe2+(aq) on the anodic regions of the metal, which are often impurities or network defects.
Elsewhere on the surface, Oxygen is reduced to water and acts as a cathode. Electrons are transferred from the anode to the cathode through the conductor metal. Water acts as a solvent for the initially produced Fe2 + and forms a salt bridge. Rust (Fe2O3 · xH2O) is formed by subsequent oxidation of Fe2+ by atmospheric Oxygen.
Iron corrosion mechanism
In the process of Ironcorrosion, Iron acts as an anode in galvanic cell and is converted to Fe2 +, Oxygen is reduced at cathodic points and converted to water.
Related reactions are as follows:
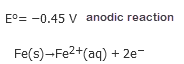


The Fe2 + ion produced in the initial reaction is then oxidized by atmospheric Oxygen to Hydrated Oxide Fe3 + according to the following reaction.
 The sign and magnitude E0 for the corrosionprocess indicate that there is a strong driving force for the oxidation of iron to oxygen under standard conditions (1MH+). In neutral conditions the driving force is slightly less but still significant (E = 1.25 V at pH 7.0).
The sign and magnitude E0 for the corrosionprocess indicate that there is a strong driving force for the oxidation of iron to oxygen under standard conditions (1MH+). In neutral conditions the driving force is slightly less but still significant (E = 1.25 V at pH 7.0).
Iron Oxidation
Oxidation of normal Iron or Steel in air and Oxygen, at a constant temperature, is rapid at first. But gradually the Oxide layer thickens, its oxidation rate also decreases.
In terms of the amount of reaction progress and the layers formation and oxides, it is usually done in a parabolic manner. So that the thickness of the oxide crust (with increasing sample weight) is a function of the time square.
The following figure shows the corresponding change curve in which the results of measurements of iron oxidation at temperatures of 700, 900 and 1100 ° C are considered in terms of time.
The presence of one or more alloying elements will cause these ratios to be disturbed. Especially, in cases where the free energy of oxide formation of these elements is greater than Iron (for example, Chromium, Silicon or Aluminum).
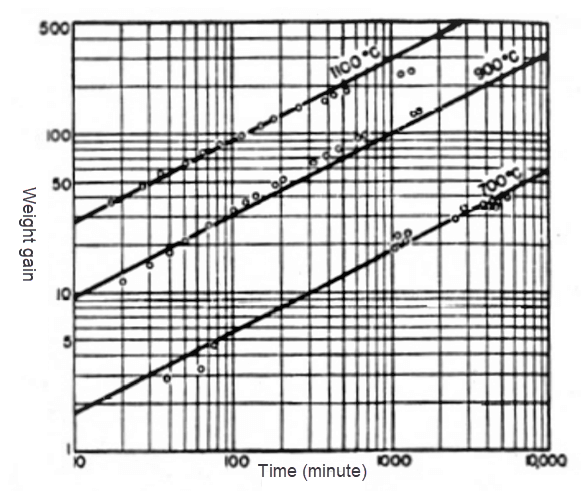
Iron oxidation is parabolic by forming three layers of iron oxide (separately). So that their ratios change with temperature and partial pressure. The reports of different researchers do not match, one of the reasons being the existence of differences in the purity of the Iron samples tested (especially in terms of carbon).
At 600 ° C and an Oxygen pressure of 11 and for 100 minutes, the resulting oxide crust is found to be two layers (consisting of an inner layer of FeO and an outer layer of Fe2 O2). At 900 degrees Celsius and for 100 minutes, the resulting oxide crust consists of three layers. So Fe2 O2 is ninety percent and FeO is less than one percent.
At below 570 ° C, the FeO layer is unstable, and if formed above this temperature, it decomposes at room temperature to Fe2 O2 and Fe.

The most suitable elements used to improve the oxidation resistance of Iron are Chromium (Cr) and Aluminum (AI). The use of these elements with amounts of Nickel and Silicon will be more effective.
Corrosion of Iron cavities
If a clean sheet of mild Steel is exposed to rain, it will rust quickly within a few days. The rust forms as hard, scaly, blistering deposits in localized areas where water droplets remain for a long time.
If we clean the rust with a wire brush, under the rust, there are cavities, which were previously covered by corrosion products.
The word rust is used here, which is the slang term for Iron corrosion. As will be explained in this section, these products are actually a combination of salts from iron corrosion. As will be explained in this section, these products are actually a combination of salts from Iron corrosion.
The effect of bacteria on iron corrosion
Organic matter in water is the result of living organisms, their metabolism, or their degradation. These organic substances are often found in the form of Carbonic acid, Humic acid, Citric acid, Acetic acid and Benzoic acid. These substances also reduce the pH of water and accelerate the corrosion of Iron.
Living organisms include bacteria, sludge, fungi and algae, and small marine oysters. Hydrogen Sulfide may be formed by Sulfate-reducing bacteria or other organisms.
They may produce corrosive Amino acids such as Cystine acid or Di-Alpha-Aminobetapropionic acid. This process has destructive effects on Iron pipes.
Iron bacteria do not directly alter water quality and do not participate in the corrosion reaction. But due to the accumulation of large amounts of ferric hydrate, it causes much more contamination than bacteria. They also block and increase friction due to the formation of bumps and create red water.
These bacteria are controlled by increasing Sodium Carbonate to raise the pH to 8.5 or increase the Chlorine. Increased Chlorine or Copper Sulfate can also be used to prevent the growth of algae.
Methods of preventing Iron corrosion
• Stainless Steels
The use of corrosion-resistant alloys is effective in preventing iron corrosion. Such as Stainless Steel.
Stainless Steels are not economically affordable for all applications of Iron, but they do cover many things.
• Lubrication
An oil-based coating helps prevent or slow down the corrosion of Iron. Because it prevents moisture from reaching its surface.
In many cases, oil can be a problem for machines and tools. It also endangers human health and the environment.
• Generate a dry cover
Dry stainless coatings that form a protective barrier layer on the metal surface. Dry coating can be useful to prevent corrosion of iron and offshore equipment and tanks.
Tin coating
Tin coating is widely used in the food industry because fruit acids do not affect tin. Food is also stored longer. This type of Iron or Steel coated with tin is called Aleppo.
This type of Iron is called Aleppo because the position of Iron in the electrochemical series is higher than Tin. With th occurrence dscratches on it, it rusts with considerable speed because Iron is relative to tin anode and is corroded.
Phosphate coatings
This coating is formed on the surface of Steel or Iron by a solution of dilute Phosphoric acid and other chemicals. The metal surface becomes a protective layer of insoluble phosphate crystals upon contact with these metal.
Iron Phosphate coating
They are in the form of blue or bluish-brown tiny crystals, whose main application is color stability.
Fluoropolymer coatings
These coatings are used to protect ferrous and non-ferrous metals from corrosive chemicals in environments up to 460 degrees Fahrenheit. These coatings have a high resistance to chemicals, which makes them suitable for a variety of applications.
• Coloring
A high quality paint slows down the rusting process by reducing access to moisture.
• Galvanize
Applying galvanized Zinc coatings on the surface of the Steel protects it from corrosion.
Zinc corrodes at a slower rate than Iron, thus protecting Iron. This is also important in terms of galvanic corrosion.
If the surface of the galvanized steel is worn or scratched, because Zinc is more active than Iron, it corrodes sooner and protects the Iron from corrosion.
• Blueing
In this process, Iron passivation is done by applying a layer of magnetite on the Iron. The metal must be lubricated regularly and in this process it will turn blue or black.
• Powder coating
An acrylic, vinyl, or epoxy coating can prevent moisture from reaching the metal surface.
• Vapor phase Corrosion Inhibitors (VCI)
Vapor barrier packages are a type of package that is easy to use, clean and dry. They are used to prevent corrosion of metal and metal components.
These inhibitors are a type of chemical compound that are injected into the environment in contact with metal. Under these conditions, they prevent the corrosion of Iron and metal by releasing Vapors that prevent corrosion of the metal.
When metal parts are properly packed with VCI, VCI is activated and steam fills the entire closed space. VCI ions form a protective layer on the metal surface that reduces corrosion.
VCI packaging without grease or oil or protective coatings safely prevents Iron corrosion.



